Finding a document collaboration system: Ideagen Document Review and SharePoint
Document collaboration platforms like Ideagen Document Review, formerly known as Ideagen PleaseReview, or SharePoint are a must-have for teams that need to write and review important documents together. The right system can break down the barriers to productivity that slow people and projects down, while freeing up time to focus on improving the quality of the content.
While SharePoint is widely known and used by teams and companies of all sizes for document collaboration, Ideagen Document Review is a more specialized solution that works seamlessly with SharePoint to deliver additional levels of control, traceability and internal/external stakeholder capabilities to take document collaboration to the next level. Both are complementary solutions that address different collaboration requirements within an organization and both are 27001 registered and provide MFA.
By understanding how the solutions can complement each other to enhance document collaboration, organizations can offer a comprehensive solution that meets the diverse needs of different departments. Here, we answer some common questions and explore some of the main ways in which the two solutions differ.
What is Ideagen Document Review?
Ideagen Document Review is a dedicated document review and co-authoring system. The web-based solution allows for easy, secure and real-time collaboration with both internal and external stakeholders. It caters to industries, such as Life Sciences, and departments like clinical research, where control, compliance and strict oversight are essential and prioritized.
What is SharePoint?
SharePoint is a web-based collaboration platform developed by Microsoft. It is primarily used for document management, storage, and sharing within organizations. SharePoint provides a centralized platform where teams can create, collaborate on, and manage documents and other content. It is well suited to teams that want a flexible and inclusive environment for collaborating on files.
What are the differing strengths of Ideagen Document Review and SharePoint?
Both systems provide a collaborative online workspace where documents can be co-authored and reviewed. However, the functionality and control levels of each system vary.
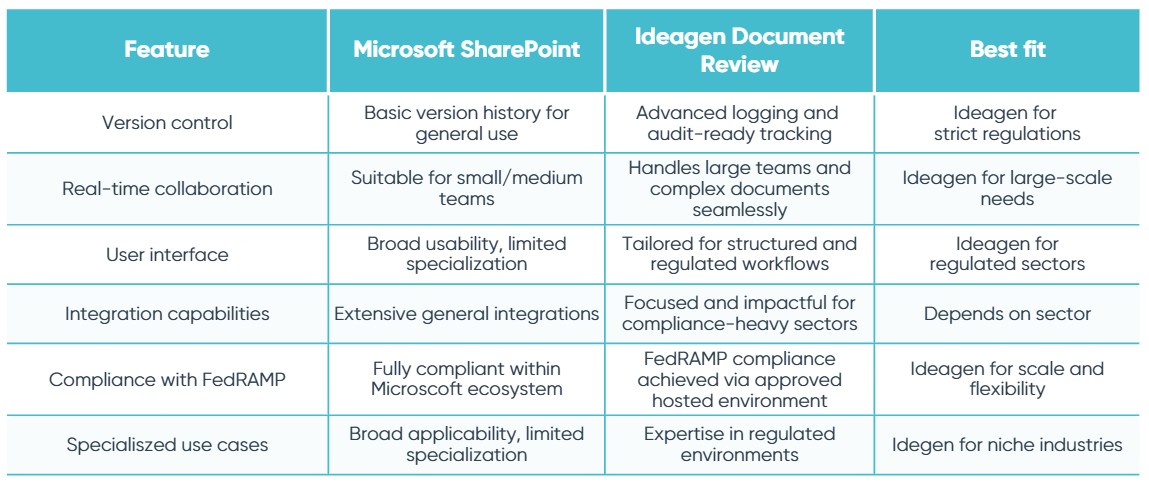
Here are the top six differences between Ideagen Document Review and SharePoint to help you better understand each system when sourcing the best document collaboration tool.
Levels of control within the document
In Ideagen Document Review, the document owner has full control over permissions and access rights, which simplifies the management process. With the use of the Zone feature, permissions are given to each person involved in the editing and construction of the document. They can be made as granular as necessary, down to individual paragraphs, and changes are accepted or rejected by the owner. Ideagen Document Review’s Zone streamlines the collaboration experience, ensuring controlled document collaboration without the need for managing multiple documents.
Ideagen Document Review automates workflows, setting pre-defined reminders, and outlines each reviewer’s progress in the ‘Review details’ page. This allows document owners to maintain total control over the review cycle, making it easier to set clearly defined start and finish points, boost reviewer accountability and ensure critical deadlines are met.
With Ideagen Document Review, you can view a complete history of the document in the one-click reconciliation report, with document comparisons for each revision. This report can serve as an ideal agenda for comment resolution meetings or color team reviews.
In contrast, users of SharePoint can turn on tracked changes and manually add comments in order to review work. There is no role distinction between co-authors and reviewers, which gives the freedom for collaborators to move in and out of their discussed remits. This can be overcome through a similar use of ‘zones’; however, the process involves manually splitting the document, assigning a user to each smaller document, and then manually merging them back to a single document after the review.
Version history
Ideagen Document Review provides robust tracking, traceability, and predefined rules for changes. This provides succinct control when several people are working on the same document at the same time, allowing for secure feedback and zero chance of being overwritten. Ideagen Document Review supports offline reviews in that comments and changes made offline are merged back into the document under review when the user goes back online. This feature supports iPad and Android tablets.
In SharePoint, everyone works on one version of the document and changes are not automatically tracked and reported. It is possible to manually turn on ‘tracked changes’ and ‘versioning’ so that previous versions of the document can be restored. SharePoint syncs local copies of files for offline viewing and editing on PC however does not merge changes made in offline/flight mode into the real-time version of the document.
Audit readiness
In Ideagen Document Review, comprehensive reporting is delivered as standard. As all activity is tracked and reportable with a reconciliation report, document owners have a full audit trail.
SharePoint does not have a report function but you are able to restore previous versions of the document.
Collaborating with third parties
In Ideagen Document Review, you can easily collaborate with people outside of your organization by simply adding them to your project. The document owner controls which documents they can access, how much of that document they can see, and what they can do within that. This control includes a redaction feature which allows for full redaction of content. This makes it a highly secure environment when working on sensitive information.
To work with third parties within SharePoint, changes to the B2B external collaboration settings in Azure Active Directory need to be made, which can be time-consuming and cumbersome. Giving external parties access to your server can pose security risks to your organization. It is common for users to share documents with third parties via email, which again poses a security risks, especially when handling sensitive documents and would require managing multiple versions of documents with different permissions.
Supporting different document formats
When collaborating across teams and with various types of content, there will likely be more than one format involved. Ideagen Document Review supports all Microsoft Office formats, as well as formats such as PDF, plain text, images and source code. This is a standard feature and involves no extra work for the user. It is also possible to review sets of documents in a single uploaded project.
As a Microsoft product, SharePoint works with Word, Excel and PowerPoint documents as standard and only Microsoft Office formats are supported.
Supporting teams of different sizes
Ideagen Document Review is stable and robust enough to support teams of two to 200+ reviewers who can all work simultaneously in the document, no matter its length or complexity. It also provides the ability to create flexible and automated review workflows for sequential, multi-phase reviews.
Microsoft indicates that co-authoring in SharePoint is designed to work for smaller teams of about 5-10 users working in the document at the same time. Due to this restriction on numbers, some organizations need to implement a process whereby different teams or individuals take turns to enter the document review.
Choosing the right document collaboration tool
SharePoint can serve as a central hub for document management and general collaboration needs, providing an inclusive and flexible environment for collaboration across your organization. Meanwhile Ideagen Document Review is a specialized tool for departments that require controlled review processes, enhanced traceability, and compliance-driven document collaboration. Users can upload documents from SharePoint into Ideagen Document Review for the review process and then check them back in after the review is completed, utilizing the integration between the two tools.
This integration bridges the gap between controlled document collaboration provided by Ideagen Document Review and the broader document management capabilities of SharePoint, offering users the advantages of both tools in a seamless workflow.
Moreover, the integration with MS Word Online ensures a consistent editing experience across platforms, allowing Ideagen Document Review to cater to a wider range of use cases in departments such as marketing, finance, and more.
Ideagen Document Review gives project owners:
- Complete control over who can view, edit and approve specific content in any document.
- Comprehensive reporting and audit trail abilities.
The solution is the ideal solution for heavily regulated industries or organizations that handle sensitive information with third parties.
Discover the benefits of Ideagen Document Review
Learn more about how Ideagen Document Review can improve your document collaboration across your organization.
James, a seasoned Product Marketer with over 30 years' experience consulting in office imaging and the document lifecycle, is responsible for our portfolio of Collaboration solutions including PleaseReview.
From the early days of PDF creation and editing, through Digital Transformation to the application of IoT and AI, he helps understand the challenges our customers experience and builds value around our solutions.
